Post translational modifications, Protein degradation and non-ribosomal protein synthesis
Post translational modifications
Many newly synthesized proteins (bacterial, archaeal, and eukaryotic) undergo various types of changes prior to their 3D folding into final biologically active conformation. These changes are know as post translational modification.
- Some common post translational modifications are:
- Amino-Terminal and Carboxyl-Terminal Modifications
- Loss of Signal Sequences
- Modification of Individual Amino Acids
- Attachment of Carbohydrate Side Chains
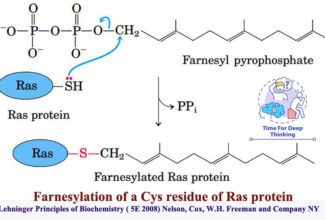
- Addition of Isoprenyl Groups
- Addition of Prosthetic Groups
- Proteolytic Processing
- Formation of Disulfide Cross-Links
Amino-Terminal and Carboxyl-Terminal Modifications
- Removal of N-formylmethionine (in bacteria) or methionine (in eukaryotes)
- In some cases, carboxyl-terminal) residues may be removed enzymatically in formation of the final functional protein.
- In as many as 50% of eukaryotic proteins, the amino group of the amino-terminal residue is N-acetylated after translation. Carboxyl-terminal residues are also sometimes modified
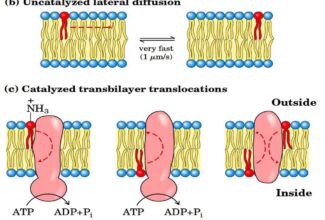
Loss of Signal Sequences
- 15 to 30 residues at the amino-terminal end of some proteins play a role in directing the protein to its ultimate destination in the cell.
- Such signal sequences are eventually removed by specific peptidase