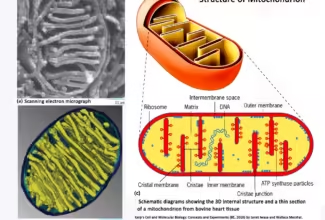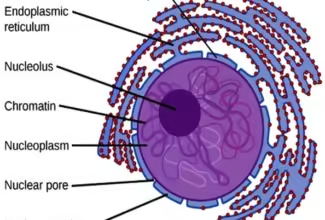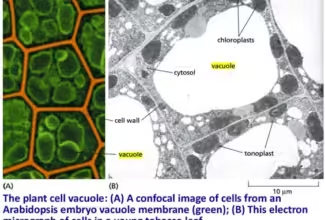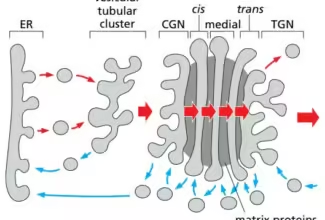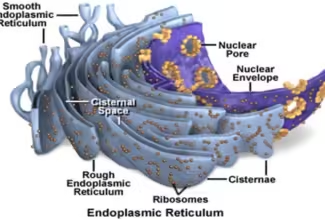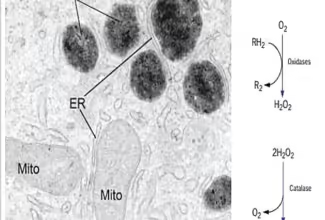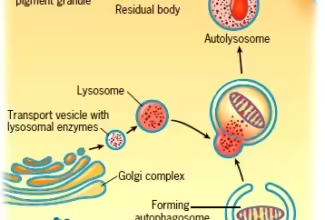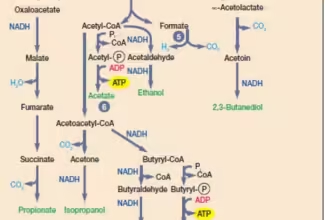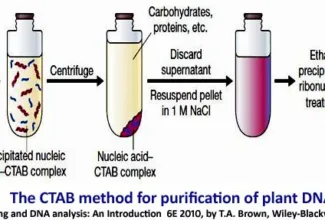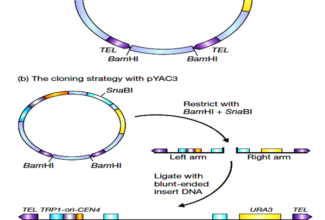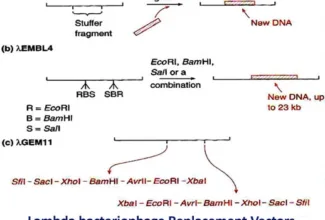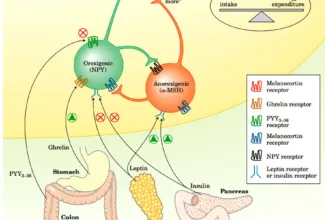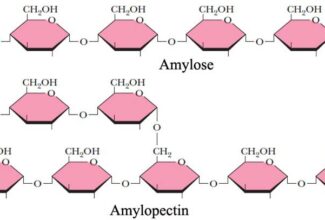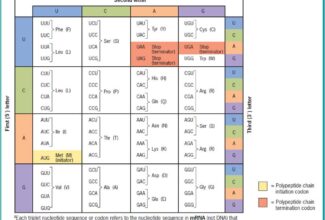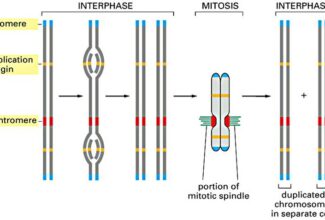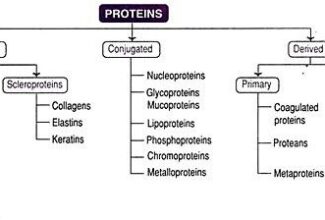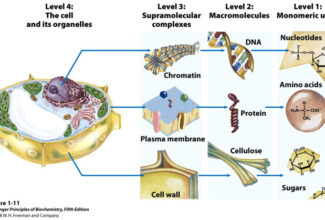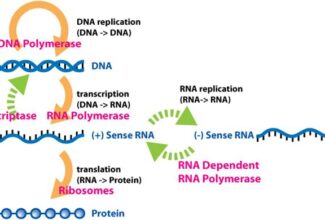
Bioenergetics: Biological Oxidation Reduction
Bioenergetics: Biological Oxidation Reduction
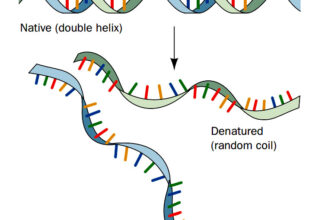
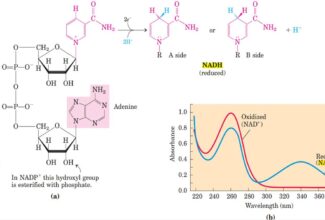
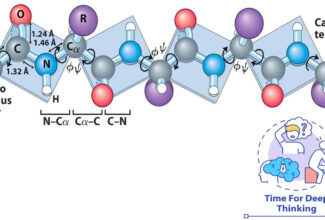
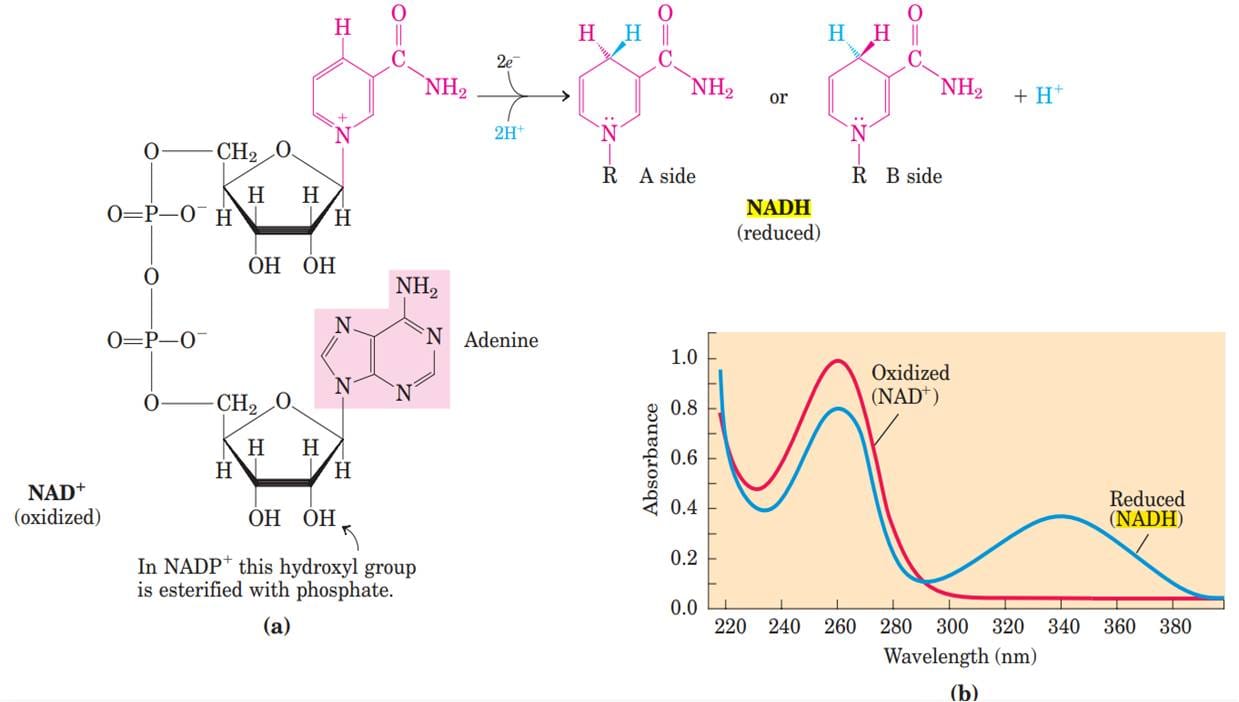
- Oxidation-reduction (redox) reactions involve the loss of electrons by one chemical species, which is thereby oxidized, and the gain of electrons by another, which is reduced.
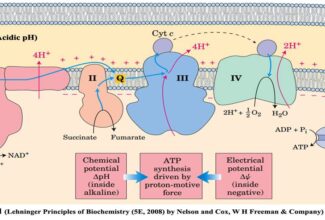
- The flow of electrons in redox reactions is responsible for all work done by living organisms.
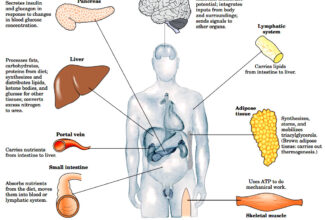
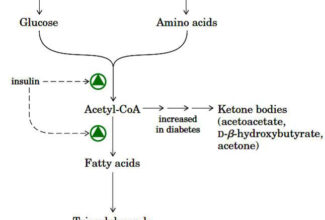
- Source of electrons: in non-photosynthetic organisms: reduced compounds (foods)
- In photosynthetic organisms, the initial electron donor: H2O in the presence sunlight.
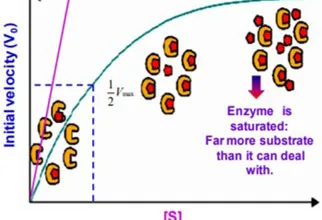
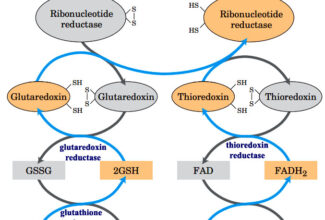
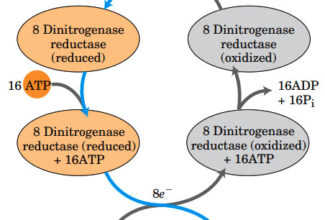
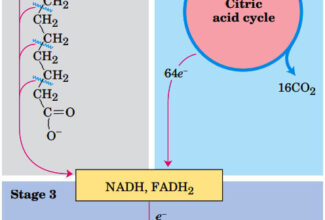
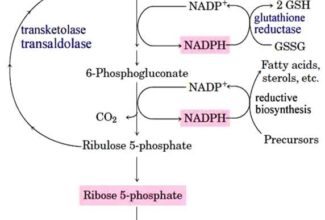
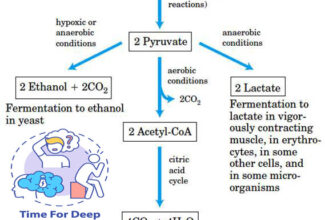
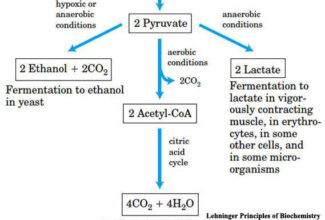
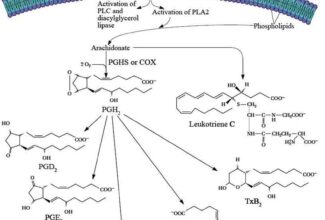
- Electrons move from various metabolic intermediates to specialized electron carriers in enzyme-catalyzed reactions. The carriers in turn donate electrons to acceptors with higher electron affinities, with the release of energy.