Bioenergetics III: High Energy Compounds
High energy compounds
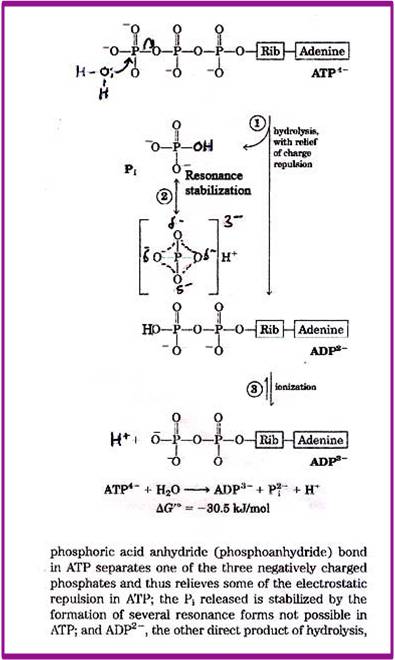
Compound that on hydrolysis undergoes a large decrease in free energy under standard conditions is called high energy compound

High energy compounds
Compound that on hydrolysis undergoes a large decrease in free energy under standard conditions is called high energy compound
July 25, 2024
July 16, 2024
July 12, 2024
July 11, 2024
July 10, 2024
June 11, 2024
April 7, 2024
March 21, 2024
March 20, 2024
March 13, 2024
March 12, 2024
March 4, 2024
February 21, 2024
February 21, 2024
February 21, 2024
February 21, 2024
January 21, 2024
January 20, 2024
January 17, 2024
January 15, 2024
January 13, 2024
January 6, 2024
December 11, 2023
December 6, 2023
December 6, 2023
October 27, 2023
October 26, 2023
October 20, 2023
October 19, 2023
October 19, 2023
October 11, 2023
October 9, 2023
October 4, 2023
October 1, 2023
June 18, 2023
June 18, 2023
March 26, 2023
February 15, 2023
December 20, 2022
December 14, 2022
December 4, 2022
November 30, 2022
November 30, 2022
November 28, 2022
November 25, 2022
November 23, 2022
November 17, 2022
September 4, 2022
June 12, 2022
May 30, 2022
May 22, 2022
May 20, 2022
May 19, 2022
May 18, 2022
May 18, 2022
May 14, 2022
May 13, 2022
May 3, 2022
April 30, 2022
April 28, 2022
April 27, 2022
April 26, 2022
April 13, 2022
April 10, 2022
April 8, 2022
March 28, 2022
March 25, 2022
March 25, 2022
March 24, 2022
March 20, 2022
March 15, 2022
March 7, 2022
February 27, 2022
February 24, 2022
February 23, 2022
February 22, 2022
February 21, 2022
February 21, 2022
February 20, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 18, 2022
February 17, 2022
February 17, 2022
January 8, 2022
January 7, 2022
January 7, 2022
January 7, 2022
January 7, 2022
January 5, 2022
January 3, 2022
January 3, 2022
January 2, 2022
January 2, 2022
January 2, 2022
January 1, 2022
January 1, 2022
January 1, 2022
January 1, 2022
December 1, 2021
December 1, 2021
November 28, 2021
November 28, 2021
November 28, 2021
November 28, 2021
November 28, 2021
November 28, 2021
October 25, 2021
October 24, 2021
October 24, 2021
October 19, 2021
October 19, 2021
October 17, 2021
October 17, 2021
October 17, 2021
October 15, 2021
October 14, 2021
October 14, 2021
October 11, 2021
October 11, 2021
October 10, 2021
October 10, 2021
