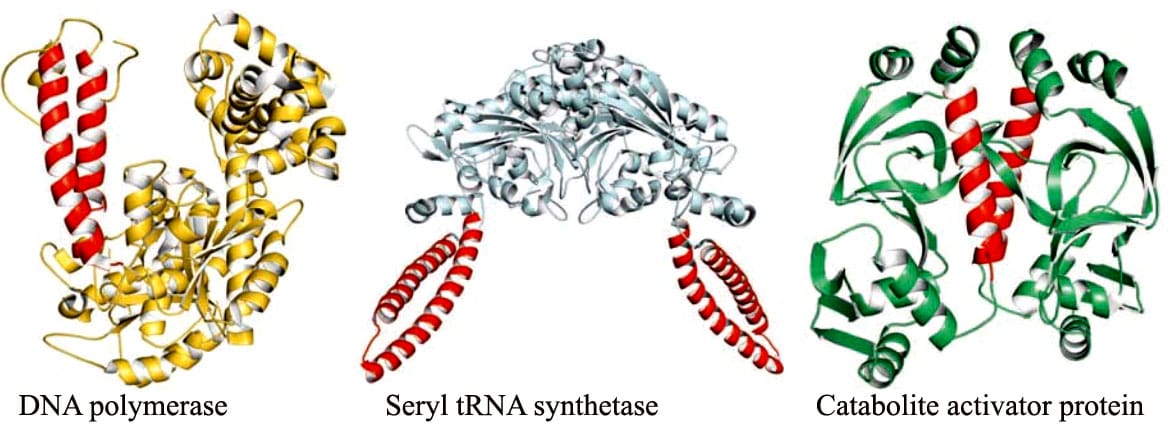
Tertiary and quaternary structures of proteins Forces stabilizing tertiary structure
Tertiary and quaternary structures of proteins Forces stabilizing tertiary structure
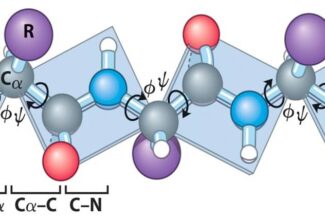
Tertiary structure refers to the complete three-dimensional structure of the polypeptide units of a given protein.
- Tertiary structure is formed due to interactions between side chain R group of amino acid residues present in the proteins.
- Nearly all of the polar, hydrophilic R groups are located in the surface, where they may interact with water
- The nonpolar, hydropobic R groups are usually burried inside the protein molecule
- In tertiary structure of proteins, covalent disulfide bonds form between closely aligned cysteine residues form the unique amino acid cystine.
- Tertiary structure is stabilized by non-covalent interactions such as hydrogen bonding, hydrophobic interactions, Van der Waal’s interactions and electrostatic interactions.
- Tertiary structure contains motifs and domains.

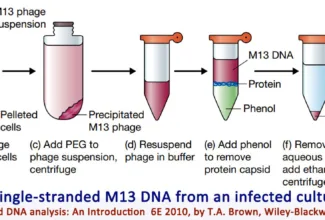
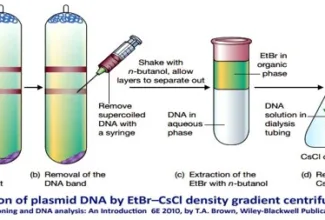

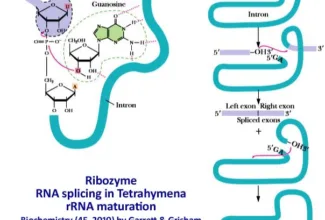
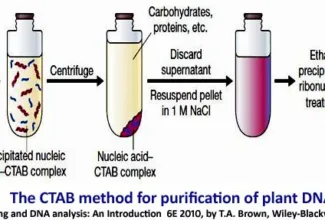


































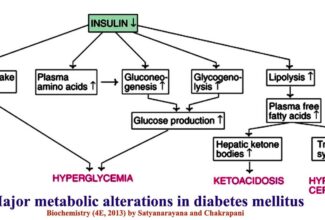


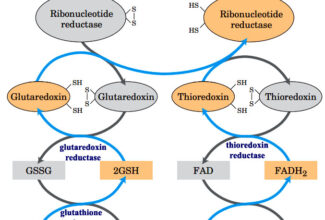
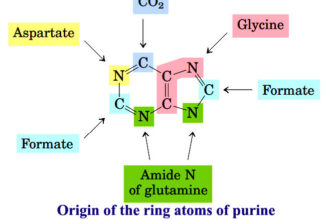












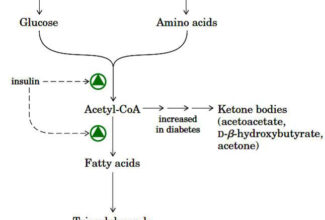























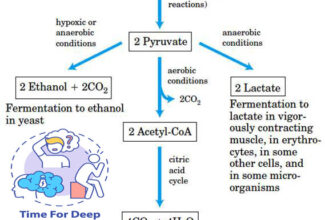
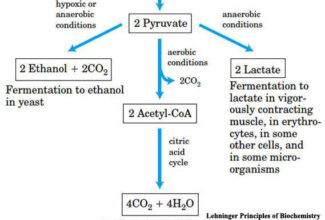













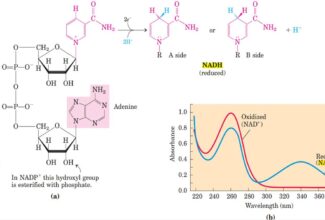

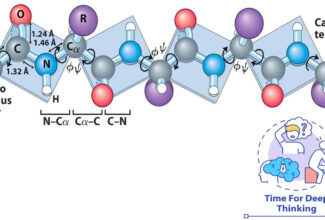








































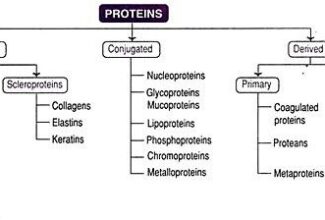





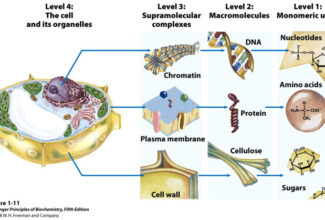
[…] Protein Structure II: Tertiary and quaternary structures of proteins; Forces stabilizing terti… […]